Chaperones in Protein Folding
January 21, 2022 2022-02-25 4:04Chaperones in Protein Folding
Chaperones are a family of proteins having functional similarities which play a vital role in the stabilization of unfolded proteins. This stabilization helps in many processes such as translocation, degradation, and folding. Chaperones protect proteins when they are undergoing the process of folding by shielding them from other proteins that might bind and hamper the process.
The chaperones system consists of molecular chaperones, co-chaperones, chaperone co-factors, and chaperone interactors and receptors that might form functional networks. Molecular chaperones are proteins that have been classified by molecular weight into different groups from the smallest (<35 kDa) to the very large (over 200 kDa). They are omnipresent; they are seen in all cells, tissues, and organs with very few exceptions. Furthermore, they are present in the various compartments of the eukaryotic cell, nucleus, cytosol, and organelles such as mitochondria and chloroplasts. Along with this, they are also present in the prokaryotic cell cytoplasm and periplasm.
Chaperones carry out their activity by redirecting aggregation towards globular, amorphous oligomers. In addition, they are often able to subdue the cytotoxic effects of protein aggregates after these are formed. They are also involved in the regulation of their own genes and in the presentation of proteins meant for degradation by proteases.
Properties of chaperones:
- Molecular chaperones interact with unfolded or partially folded protein subunits, e.g., nascent chains emerging from the ribosome or extended chains being translocated throughout subcellular membranes
- They do not interact with native proteins, nor they form part of the final folded structures
- Some chaperones are non-specific and interact with a wide variety of polypeptide chains but others are restricted to specific targets
- Essential for viability, their expression usually increases with cellular stress
Types of chaperones:
There are several families of chaperones and each possesses different functions. Although constitutively expressed under balanced growth conditions, many chaperones are triggered upon heat shock or other stresses that increase cellular protein misfolding (including heterologous protein expression) and are thus classified as stress or heat shock proteins (Hsps). Molecular chaperones have been divided into three functional subclasses based on their mode of action. ‘Folding’ chaperones e.g., DnaK and GroEL depend on ATP-driven conformational changes to bring about the net folding/unfolding of their substrates. ‘Holding’ chaperones e.g., Hsp33, Hsp31, and IbpB maintain partially folded proteins on their surface in order for the folding chaperones to be available. And the third type of chaperone e.g., ClpB promotes the solubilization of proteins that have become aggregated as a result of stress. The name Hsp- heat shock proteins were given after these proteins were discovered in a bacterium. These bacteria produced a greater number of these proteins in stressful conditions like high temperature, pH variation, and hypoxic conditions.
Hsp70:
The Hsp70 chaperone proteins are folding stimulators that aid in many kinds of folding processes such as refolding of misfolded aggregated proteins, folding and assembling of new proteins. These proteins are monomeric and contain N and C terminals. The N terminal has ATPase while the C terminal is responsible for binding with the substrate. The C terminal opens and binds to the substrate when ATP hydrolysis occurs at the N terminal. Hsp70 recognizes the extended region of an unfolded polypeptide; this extended region consists of many hydrophobic residues. The binding of Hsp70 prevents the aggregation of these proteins.
Hsp60:
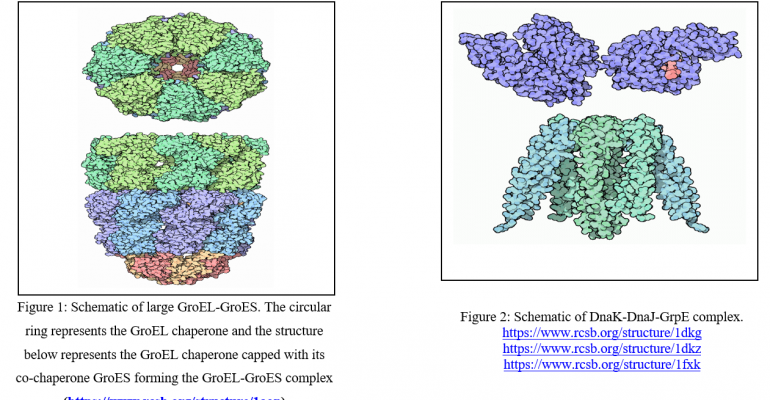
Similar to Hsp70, Hsp60 chaperone proteins are also capable of binding to exposed hydrophobic residues to form aggregates that are stable but inactive. These proteins do not take part in preventing aggregation, but instead, function to quarantine and isolate unfolded proteins. This activity of isolation prevents the polypeptide chain from aggregating into clumps with other chains within the cytoplasm. Hsp60 contains 14 different protein components. These proteins form two rings made of 7 proteins each of which are placed on top of each other. Unfolded proteins are then able to fold without aggregating with other unfolded proteins within these rings and without the interference from Hsp70.
REFERENCES
1. Paladino, L. et al. The Role of Molecular Chaperones in Virus Infection and Implications for Understanding and Treating COVID-19. J. Clin. Med. 9, 3518 (2020).
2. François Baneyx. 2008.pdf.
3. Goodsell, D. S. Chaperones. RCSB Protein Data Bank (2002) doi:10.2210/rcsb_pdb/mom_2002_8.
4. Allen, W. J., Phan, G. & Waksman, G. Structural biology of periplasmic chaperones. Advances in protein chemistry and structural biology vol. 78 (Elsevier, 2009).
5. Kaufman, R. J. & Popolo, L. Protein Synthesis, Processing, and Trafficking. Hematology: Basic Principles and Practice (Elsevier Inc., 2018). doi:10.1016/B978-0-323-35762-3.00005-6.
6. Macario, A. J. L. & Conway de Macario, E. Chaperone Proteins and Chaperonopathies. in Encyclopedia of Stress (Second Edition) (ed. Fink, G.) 438–444 (Academic Press, 2007). doi:https://doi.org/10.1016/B978-012373947-6.00075-1.
7. Breydo, L., Redington, J. M. & Uversky, V. N. Effects of Intrinsic and Extrinsic Factors on Aggregation of Physiologically Important Intrinsically Disordered Proteins. International Review of Cell and Molecular Biology vol. 329 (Elsevier Inc., 2017).
8. Simmons, H. What are Chaperone Proteins? News Med. Life Sci. 1–5 (2019).
Authored by Gandhali Vichare, Batch 20, Biocon KGI Certificate Program in Biosciences